
Step 1: Subtract the accepted value from the experimental value. Copper’s accepted density is 8.96 g/cm 3. When you calculate the density using your measurements, you get 8.78 grams/cm 3. In the present study we address these points in the use.
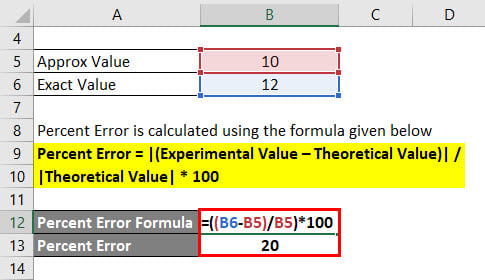
You measure the sides of the cube to find the volume and weigh it to find its mass. Moreover, little is known about the power of percentage prediction error as statistical inference.
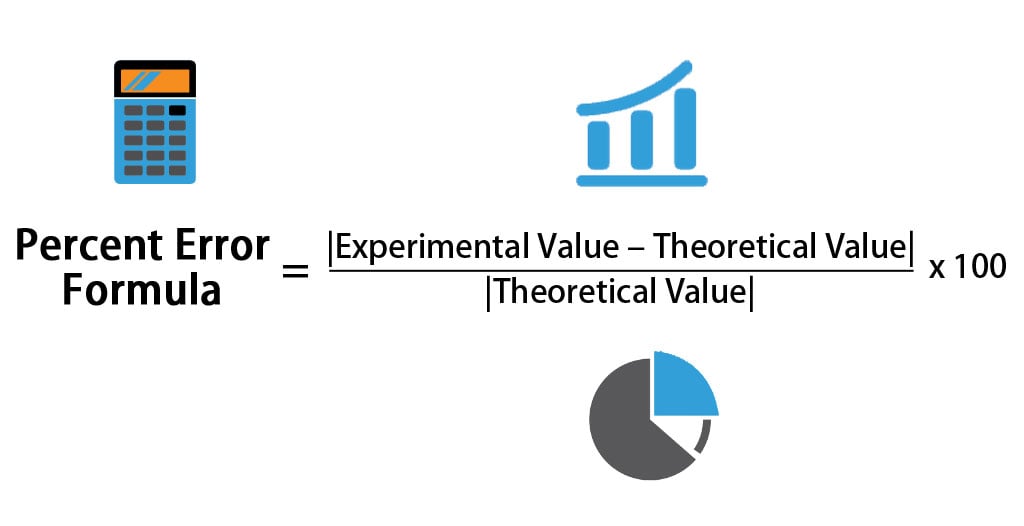
For example, in experiments involving yields in chemical reactions, it is unlikely you will obtain more product than theoretically possible. If you need to know the positive or negative error, this is done by dropping the absolute value brackets in the formula. In most cases, absolute error is fine. Note: occasionally, it is useful to know if the error is positive or negative. Numerical Methods.The formula for calculating percent error is: Percentage error = 2.174 % Percentage Error Example 2 We measure the temperature of a surface 45 degree Celsius and the theatrical temperature of the surface was calculated 46 degree Celsius. Relative error The relative error in any calculation is the absolute error divided by the actual value. Now to calculate the percent error, we will divide it by 20. Percentage error = 14.286 % Percentage Error Example 1 In the above example, the absolute error is 0.02.


 0 kommentar(er)
0 kommentar(er)
